Abstract
Background: Aging population has resulted in an increase in the incidence of acute myeloid leukemia (AML). The median age of diagnosis for AML is 68 years. Allogeneic hematopoietic cell transplant (alloHCT) is a curative modality for patients with AML, however, disease relapse remains a major issue. Human leukocyte antigen (HLA)-matched sibling donors (MSD) are considered the preferred donor type in clinical practice. However, with increasing median age of patients, sibling donors for these patients are likely to be older as well, often with higher comorbidities. Prior registry studies demonstrated that older donor age is associated with inferior survival. A recent study in older MDS patients demonstrated reduced relapse risk with improved disease-free survival (DFS) with younger matched unrelated donors (MUD). However, the impact of donor age and type on alloHCT outcomes in AML patients remains unknown.
Methods: This retrospective cohort study accessed data from the Center for International Blood and Marrow Transplant Research database (CIBMTR) in AML patients 50 years or older undergoing alloHCT from older (>50) MSDs or younger (<35) MUDs between 2011 and 2018. The study included common allograft types, conditioning regimens, and graft-versus-host-disease (GVHD) prophylaxis strategies. Other exclusion criteria were recipients of ex vivo T-cell depleted grafts, mismatched unrelated donor, cord blood or identical twin transplants. The primary outcome, compared between the 2 donor age groups, was relapse risk, and secondary outcomes included non-relapse mortality (NRM), DFS, and overall survival (OS).
Cumulative incidence estimates were calculated for competing risks outcomes. To evaluate potential risk factors, multivariable cox regression was used, and relevant transplant-related covariates were considered. Interactions between the main effect (donor age group) and significant risk factors were tested. Fine and gray model was used for NRM and relapse.
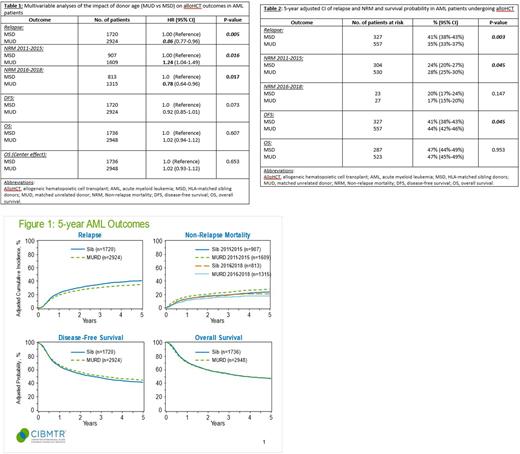
Results: Among 4684 eligible patients in the study cohort, 1736 underwent alloHCT with an older MSD (median age, 61 [range: 50-80]) whereas 2948 received transplant from a younger MUD (median age, 63 [range: 50-84]). In multivariable analysis, younger MUDs conferred a significantly decreased risk of relapse (HR 0.86; 95% CI, 0.77-0.96; p=.005) as compared to older MSDs (Table 1; Figure 1). The adjusted cumulative incidence of relapse at 5 years was significantly lower with younger MUDs (35%; 95% CI, 33-37%) versus older MSDs (41%; 95% CI, 38%-43%) (p=.003) (Table 2). NRM was reported in 2 subgroups of transplant years (2011-2015 and 2016-2018) as there was significant interaction between the main effect (MUD vs MSD) and alloHCT year (p=.003). Younger MUDs conferred an increased NRM (HR 1.24; 95% CI, 1.04-1.49; p=.016) compared to older MSDs in the earlier period of 2011-2015, whereas the NRM was lower with younger MUDs in the latter period of 2016-2018 (HR, 0.78, 95% CI, 0.64-0.96; p=.017) (Table1; Figure 1). While there was no difference in the adjusted cumulative incidence of NRM at 5 years in the latter period (17% vs 20%; p=.147), NRM was significantly higher in AML patients who underwent alloHCT between 2011 and 2015 from a younger MUD (28%; 95% CI, 25-30%) compared to older MSDs (24%; 95% CI, 20%-27%; p=.045) (Table2). There was no difference in OS or DFS rates of alloHCT with younger MUDs vs older MSDs (OS: HR 1.02; 95% CI, 0.94-1.12; p=.607; DFS: HR 0.92; 95% CI, 0.85-1.01; p=.073) (Table 1; Figure 1). While the adjusted 5-year OS probability was 47% in each of the 2 cohorts (p=0.95), the adjusted 5-year DFS probability was 44% with younger MUDs (95% CI, 42%-46%) as compared to 41% with older MSDs (95% CI, 38%-43%; p=.04) (Table2).
Conclusions: This large study from a prospectively collected registry data found significant relapse reduction in AML patients who underwent alloHCT with younger MUDs as compared to older MSDs and highlights that a younger MUD donor type exerts a stronger GVL effect and should be preferred when available. The higher NRM associated with younger MUDs has decreased in recent years, likely due to enhanced GVHD prophylaxis, infection preemption and preventative strategies, and supportive cares measures translating into improved leukemia-free survival. The results aid in donor selection and younger MUDs should be preferred in AML patients at a higher risk for post-alloHCT relapse.
Disclosures
Zhang:Gamida Cell: Research Funding; Bristol Myers Squibb: Research Funding; Astellas Pharma Inc: Research Funding. Sabloff:Roche: Membership on an entity's Board of Directors or advisory committees; Actinium: Membership on an entity's Board of Directors or advisory committees; Astellas Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Taiho Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board. Kebriaei:Ziopharm: Research Funding; Amgen: Research Funding; Kite: Consultancy; Pfizer: Consultancy; Jazz: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal